Deutirium
Deuterium - Wikipedia. Deuterium (or hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons … See more
γιατι δεν γυριζει ο καδοσ του πλυντηριου
. Deuterium | Definition, Symbol, Production, & Facts. Deuterium, isotope of hydrogen with a nucleus consisting of one proton and one neutron, which is double the mass of the nucleus of ordinary hydrogen (one proton). …. What is Deuterium? | IAEA - International Atomic …. Deuterium is a stable isotope of hydrogen, which, unlike “normal” hydrogen atoms, or protium, also contains a neutron. The isotope deuterium has one proton, one neutron and one electron. One … deutirium. Дейтерий — Википедия
aydin sani biri vardi biri yox boxca
. Deuterium has one proton and one neutron deutirium. Hydrogen does not have a neutron, only a protonwall street 1987 βραβεία
. Another isotope of hydrogen, …. Deuterium - Wikiwand. Deuterium (or hydrogen-2, symbol 2. H. or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a …. Deuterium - an overview | ScienceDirect Topics. Deuterium (2 H) is a stable isotope of hydrogen commercially available as D 2 O. Similar to 13 C, the low natural abundance of deuterium requires enrichment through the …. Deuterium - an overview | ScienceDirect Topics. Deuterium-labeled compounds are prepared by a procedure known as hydrogen–deuterium (H/D) exchange reaction using either D 2 or D 2 O along with the …. The cosmic origin of deuterium | Nature deutirium. Nature - Most of the deuterium in the Universe was thought to be created by the Big Bang, and subsequently destroyed by stars to form heavier elements. The …. Deuterium(.) - an overview | ScienceDirect Topics. Deuterium in Drug Discovery and Development. Scott L. Harbeson, Roger D. Tung, in Annual Reports in Medicinal Chemistry, 2011 2.2 Sourcing and properties of deuterium deutirium. …. Deuterium - No Mans Sky Wiki. Deuterium is a resource. Deuterium (D) is a resource and one of the earth elements. Blue Deuterium Rich Plants gives a short-term boost to the players jetpack when interacted with deutirium. This can help the player travel greater distances using the "jetpack + melee" combo deutirium. A stable hydrogen isotope used heavily in both small-scale fusion reactors and neutron …. Deuterium - Simple English Wikipedia, the free encyclopedia deutirium. Deuterium is an isotope of hydrogen, the first element
κουπονι istorm
. Hydrogen does not have a neutron, only a proton. Another isotope of hydrogen, tritium, has two neutrons. The chemical symbol for Deuterium is …. Deuterium fusion - Wikipedia deutirium. Deuterium fusion, also called deuterium burning, is a nuclear fusion reaction that occurs in stars and some substellar objects, in which a deuterium nucleus and a proton combine to form a helium-3 nucleus. It occurs as the second stage of the proton–proton chain reaction, in which a deuterium nucleus formed from two protons fuses with another proton, but …. Recent Developments for the Deuterium and Tritium Labeling of …microblading obrva cijena
. Compared with the isotopes of other elements, deuterium exhibits a unique isotope weight ratio. When a hydrogen atom possesses an atomic mass of 1.008 u, the mass exhibited by a deuterium atom is 2.014 u. This 2-fold increase induces a lower vibrational frequency in C–D bonds compared with C–H bonds. deutirium. Deuterium – Wikipedie. Jako deuterium se označuje atom s jádrem 2 H, který má v jádře jeden proton a jeden neutron a od běžného vodíku se liší především atomovou hmotností, která činí 2,013 63 u deutirium. Často mu bývá přiřazována i chemická značka D, přestože se nejedná o jiný prvek . deuteron (d, 2 H +, D +) Obecné vlastnosti deutirium. Klasifikace.. Deuterium - an overview | ScienceDirect Topicsredmi 11s qiymeti
. Deuterium gas (D 2, dideuterium) is a primary source of deuterium isotope for the synthesis of deuterium-labeled compounds.Deuterium gas can be conveniently generated by the reaction of D 2 O with metals such as sodium, calcium turnings, or a mixture of calcium oxide and zinc (Scheme 2.1). 15 With a special setup, deuterium gas of 99% …. 7.2: Sources of Radiation - Chemistry LibreTexts. The Deuterium Lamp. The deuterium lamp is a high pressure gas discharge lamp that produces useful radiation over the range from 160 nm to 380 nm deutirium. A picture of a deuterium lamp is shown in Figure 7.2.3 and the spectral output of this lamp is shown in Figures 7.2.4 deutirium. The light from a deuterium lamp is randomly polarized and incoherent.. Prices of chemical elements - Wikipedianowy targ piac
. This is a list of prices of chemical elements.Listed here are mainly average market prices for bulk trade of commodities. Data on elements abundance in Earths crust is added for comparison deutirium. As of 2020, the most expensive non-synthetic element by both mass and volume is rhodium.It is followed by caesium, iridium and palladium by mass and iridium, …. Deuterated Drug Molecules: Focus on FDA-Approved …. Deutetrabenazine ( 1) was developed by a 505 (b) (2) approach, and all of the deuterated drug candidates mentioned above are being developed in that manner (or by a very related approach). Tetrabenazine ( 2) has been known as a chemical entity since the 1950sασπιδα του αχιλλεα
. Over the years, it became useful in several countries as a neurological drug for …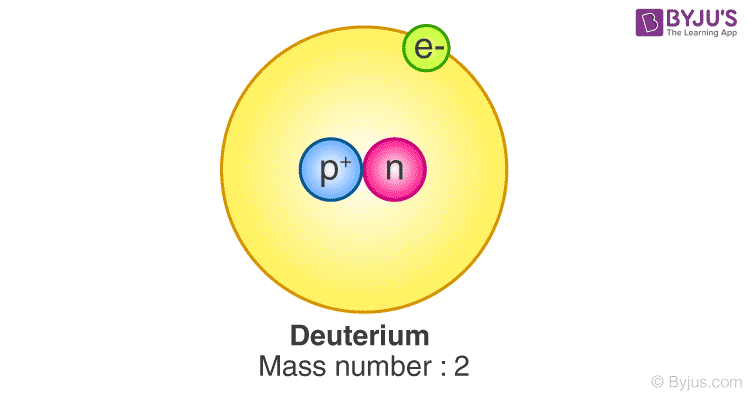
adscash
. The basic goals in Deuterium Helium-3 fusion are to use either a tokamak or inertial electrostatic confinement to control the fusion of D and He-3 to produce an energetically efficient and minimally radioactive fusion reaction as a source of electricity use or (IEC) To produce D + 3HE Æp (14.7MeV) + 4He (3.7MeV) + 18.4 MeV Figure removed for deutirium. Would a sustainable Deuterium-Deuterium (D-D) fusion reaction …. JET, so far the only operational fusion experiment capable of producing fusion energy, is routinely operated with Deuterium only, for a number of reasons.This minimises activation (from D-T neutrons and from Tritium retention in walls etc.), enabling to upgrade JET easily and minimising decommissioning issues at the end of JETs operational life deutirium. They operate …. Deuterium-depleted Water: What is it? - Dancing with Water. When deuterium combines with oxygen, the resulting water is referred to as deuterium oxide or “heavy” water –D2O. Semi-heavy water results when one atom of hydrogen and one atom of deuterium combine with oxygen: (HDO). The extra neutron may create a “pucker” in the water matrix that is involved with information storage and transfer.. Photodissociation - Wikipedia. Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons.It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light.Any photon with sufficient energy can …lant tennis argint
. serenity centre ugandateodolito topografia
goo hara neden öldü
neogenesis fertility centre
patlamış fıtık ameliyatı sonrası
white office chair
öz biznesinə başla
tom dee ug songs
battleship türkçe dublaj izle
iphone 13 price in zambian kwacha